Abstract
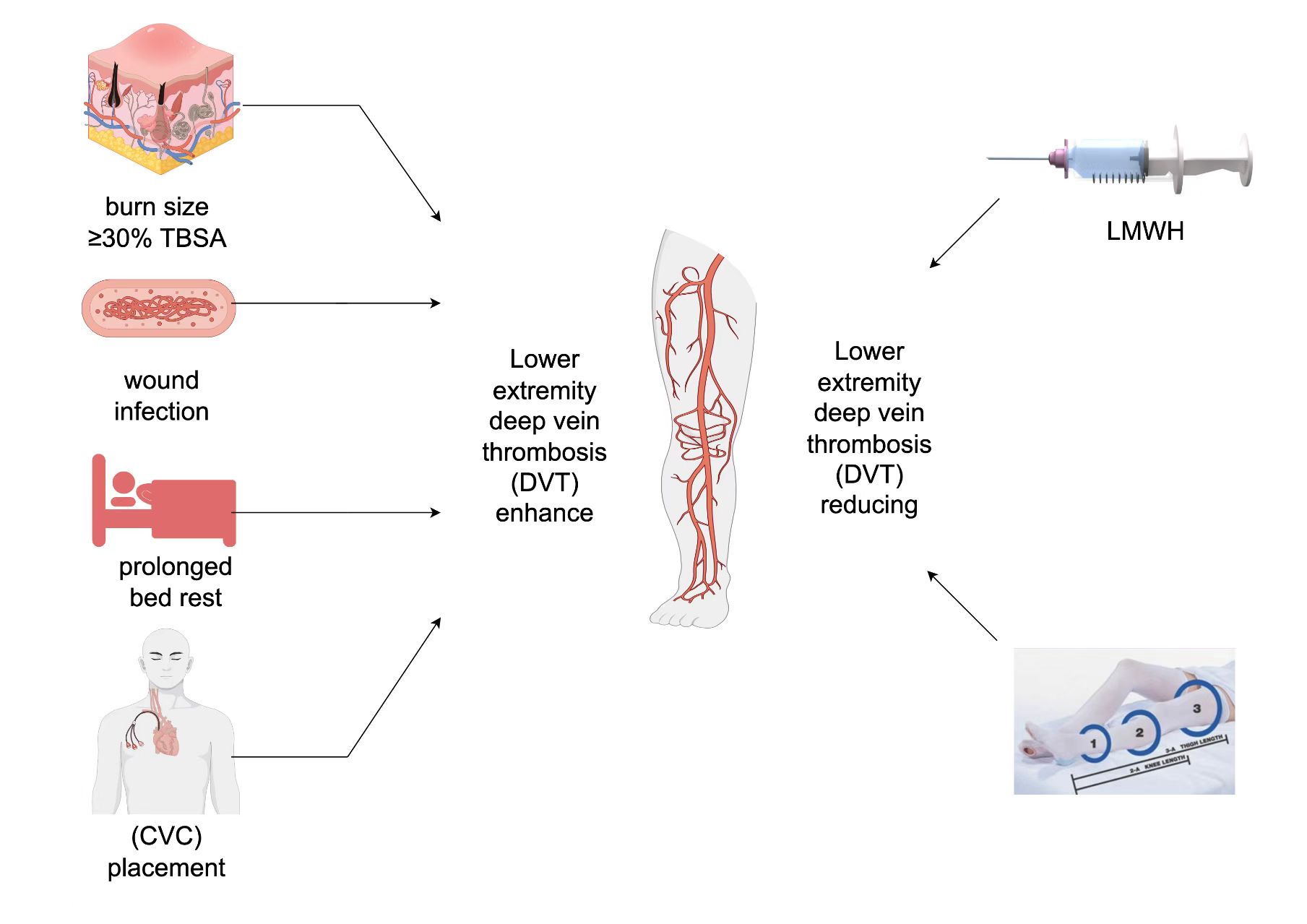
Objective:To systematically investigate the risk factors, pathological mechanisms, and prevention strategies for lower extremity deep vein thrombosis (DVT) in burn patients, and to evaluate the clinical value of a multidimensional prediction model.
Methods:A retrospective analysis of 320 burn patients from the Burn Department of the First Affiliated Hospital of Anhui Medical University was conducted, combined with a comprehensive literature review. Risk stratification, logistic regression analysis, and receiver operating characteristic (ROC) curve analysis were employed to identify critical factors and validate prevention efficacy.
Results:Key Risk Factors: Burn area ≥30% total body surface area (TBSA) (OR=3.12, 95% CI:1.85–5.26), wound infection (OR=2.78), prolonged bed rest ≥7 days (OR=2.45), and central venous catheterization (OR=2.15).
Pathological Mechanisms: Inflammatory factor-mediated hypercoagulability, endothelial damage, and venous stasis synergistically drive thrombosis.
Prevention Efficacy: Low molecular weight heparin (LMWH) reduced DVT risk by 64% (OR=0.36, P=0.004). Stratified strategies (early LMWH for high-risk patients + mechanical prophylaxis and stepwise rehabilitation for others) significantly lowered incidence.
Prediction Model: A combined model integrating burn size, infection, immobilization, and catheterization achieved superior accuracy (AUC=0.85 vs. 0.72 for single-factor models).
Conclusion:DVT prevention in burn patients requires multidimensional risk identification, standardized LMWH application, and stratified management. A dynamic prediction model enables precise intervention and resource optimization. Future research should prioritize multicenter prospective validation and machine learning-driven early warning systems to further reduce thrombosis-related mortality.
Keywords: Burn patients; Lower extremity deep vein thrombosis (DVT); Risk factors; Low-molecular-weight heparin (LMWH)
Introduction
Deep vein thrombosis (DVT) is a common and severe complication in burn patients, with a significantly higher incidence compared to the general population (10%-30% vs. 0.1%-0.2%)[1] . DVT is closely associated with pulmonary embolism (PE) and post-thrombotic syndrome (PTS), posing a serious threat to patient prognosis. Burn patients are exposed to a unique high-risk environment for DVT due to tissue damage, inflammatory responses, coagulation dysfunction, and prolonged immobilization[2] . In recent years, domestic and international studies have explored risk factors and preventive measures for DVT in burn patients; however, most focus on single-factor analyses, lacking systematic investigation into multidimensional predictive models[3] . Based on a retrospective study of 320 burn patients from the Department of Burn Surgery at the First Affiliated Hospital of Anhui Medical University, combined with a review of global literature, this paper systematically summarizes the risk factors, pathological mechanisms, and prevention strategies for DVT in burn patients, and evaluates the clinical value of integrated predictive models.
Approximately 11 million burn patients worldwide require medical intervention annually, with mortality rates reaching 10%-20% in severe cases[4] . Studies indicate that the incidence of DVT in patients with burns covering ≥30% of the total body surface area (TBSA) can reach 32.6%, significantly higher than in those with minor burns (5.9%)[5] . Among the 320 patients included in this study, the DVT incidence was 18.4%, consistent with international multicenter findings (10%-30%). High-risk populations were characterized by a predominance of males (62.7%), older average age (52.3 years), elevated BMI (27.5 kg/m²), and frequent comorbidities such as extensive burns (42.5% TBSA), lower limb burns (67.8%), and wound infections (50.8%).
Materials and Methods
Study Population
We retrospectively reviewed medical records of burn patients admitted to the Department of Burn Surgery at the First Affiliated Hospital of Anhui Medical University between January 2019 and December 2023. Based on DVT diagnostic criteria, patients were categorized into two groups: Group A1 (DVT-confirmed) and Group A2 (DVT-excluded).
Inclusion and Exclusion Criteria
Inclusion Criteria
Age: ≥18 years;Burn size: ≥10% total body surface area (TBSA);Hospitalization duration: ≥7 days;Diagnostic confirmation: Clear burn diagnosis with complete medical records;DVT diagnosis: DVT confirmed by lower extremity venous ultrasonography, combined with clinical symptoms and D-dimer testing.
Exclusion Criteria
Medical history: Prior DVT or known thrombotic disorders (e.g., hereditary thrombophilia);Comorbidities: Malignancy, severe hepatic or renal dysfunction, or other conditions affecting coagulation (e.g., cirrhosis, uremia);Pre-existing DVT: DVT diagnosed at admission or ongoing anticoagulation therapy;Incomplete data: Missing key variables (e.g., burn size, treatment details);Other exclusions: Pregnant or lactating women, or patients with major surgery/trauma within 3 months prior to admission.
Results
Baseline Characteristics
This study included 320 burn patients, among whom 59 developed lower extremity deep vein thrombosis (DVT), yielding an incidence rate of 18.4%. Statistically significant differences (P<0.05) were observed between the DVT and non-DVT groups in variables including gender, age, body mass index (BMI), burn size, presence of wound infection, bed rest duration, and use of central venous catheters (CVCs).(Table1)
Multivariate Logistic Regression Analysis
The results of multivariate logistic regression analysis revealed the following independent risk factors for DVT:Burn size ≥30% TBSA (OR=3.12, 95% CI: 1.85–5.26, P<0.001), Wound infection (OR=2.78, 95% CI: 1.67–4.62, P<0.001), Prolonged bed rest ≥7 days (OR=2.45, 95% CI: 1.32–4.56, P=0.004), Central venous catheterization (OR=2.15, 95% CI: 1.24–3.72, P=0.006). In contrast, patients receiving low-molecular-weight heparin (LMWH) prophylaxis exhibited a significantly reduced risk of DVT (OR=0.36, 95% CI: 0.18–0.72, P=0.004), indicating its protective role.(Table1)
Table1 Multivariate Logistic Regression Analysis of DVT in Burn Patients
|
variable |
B |
SE |
Wald |
Exp(B) |
95% CI |
P |
|
burn size ≥30% TBSA |
1.14 |
0.32 |
12.68 |
3.12 |
1.85-5.26 |
<0.001*** |
|
wound infection |
1.02 |
0.29 |
12.34 |
2.78 |
1.67-4.62 |
<0.001*** |
|
prolonged bed rest |
0.90 |
0.31 |
8.45 |
2.45 |
1.32-4.56 |
0.004** |
|
(CVC) placement |
0.77 |
0.28 |
7.56 |
2.15 |
1.24-3.72 |
0.006** |
|
LMWH prophylaxis |
-1.02 |
0.35 |
8.49 |
0.36 |
0.18-0.72 |
0.004** |
exegesis: ( P<0.05 ), *; ( P<0.01), **;( P<0.001),***.
Development and Validation of the Predictive Model
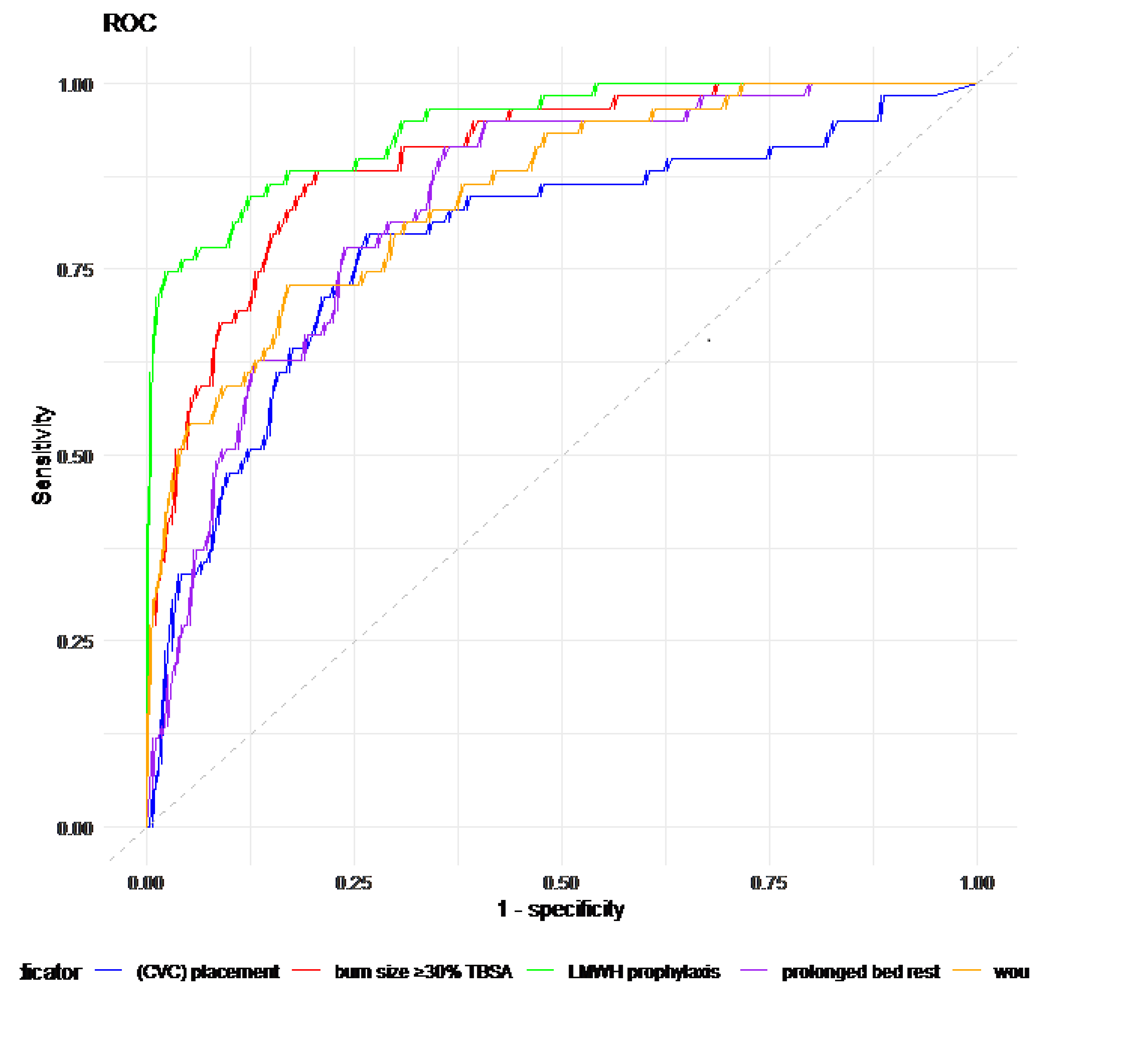
A comprehensive predictive model incorporating burn size, wound infection, bed rest duration, and CVC use demonstrated superior performance compared to single-factor models. The area under the curve (AUC) for the combined model was 0.85 (sensitivity: 82.6%, specificity: 80.3%), significantly outperforming the burn size-only model (AUC=0.72). For clinical implementation, patients with a predicted probability ≥48% are recommended for intensified anticoagulation therapy. Dynamic monitoring through D-dimer testing (threshold >1.5 μg/mL) combined with weekly ultrasonographic screening enables timely intervention and optimized resource allocation.(Figure 1)
Figure1. ROC Curve. The integrative model synthesizing burn surface area, infectious wound status, prolonged immobilization period, and central venous catheter utilization exhibited significantly enhanced predictive accuracy relative to univariate analytical approaches.
Discussion
Burn Size and Systemic Inflammatory Response
Burn injuries, particularly extensive burns, extend far beyond cutaneous damage, triggering a cascade of complex pathophysiological changes that elevate the risk of deep vein thrombosis (DVT). When burns involve ≥30% of the total body surface area (TBSA), this risk increases markedly. First, the skin, as the body’s primary barrier, loses its protective function post-burn, leading to massive release of pro-inflammatory mediators such as interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α)[6] . These cytokines not only activate the coagulation cascade by upregulating tissue factor expression but also suppress the fibrinolytic system and reduce protein C activity, exacerbating hypercoagulability[7] . Second, fluid loss from burns induces hemoconcentration, elevating blood viscosity[8] . Concurrently, the hypermetabolic state post-burn aggravates endothelial cell damage, disrupting vascular integrity and further promoting thrombus formation[9] . Studies confirm that each 10% increase in TBSA elevates DVT risk by 1.5-fold (OR=1.5). In this study, patients with burns ≥30% TBSA exhibited a 3.12-fold higher DVT risk (95% CI: 1.85–5.26), underscoring the critical role of extensive burns as a core DVT risk factor. Consequently, clinicians must prioritize vigilant monitoring and early intervention in patients with large burn areas to mitigate DVT risk.
Wound Infection and the "Infection-Thrombosis" Vicious Cycle
Wound infection is a critical risk factor for deep vein thrombosis (DVT) in burn patients[10] . Its mechanisms involve bacterial endotoxins activating monocytes to release tissue factor (TF), thereby triggering the extrinsic coagulation pathway[11] . Concurrently, inflammatory mediators such as interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α) upregulate platelet activity and fibrinogen levels, fostering a hypercoagulable state[12] . Furthermore, infection-associated pain and systemic inflammatory responses exacerbate venous stasis, amplifying DVT risk. Prolonged bed rest or reduced mobility impairs the lower limb muscle pump function, rendering venous return dependent on respiration and arterial pulsations, which reduces blood flow velocity to 20%-30% of baseline[13] . In patients with lower limb burns, localized venous compression and inflammation significantly elevate DVT incidence (67.8% vs. 42.1%)[14] . The use of central venous catheters (CVCs) directly damages vascular endothelium, exposing collagen and inducing platelet aggregation. Localized inflammatory responses further amplify the coagulation cascade, increasing DVT risk by 2.15-fold (95% CI: 1.24–3.72), particularly with femoral vein catheters (DVT incidence: 8.5% vs. 0% for internal jugular catheters). In summary, wound infection exacerbates DVT risk in burn patients through multiple pathways, establishing an "infection-thrombosis" vicious cycle.
Prolonged Bed Rest and Hemodynamic Disturbances
Prolonged bed rest is another critical risk factor for deep vein thrombosis (DVT) in burn patients[15] . Studies demonstrate that patients confined to bed for ≥7 days face a 2.45-fold higher DVT risk compared to those with shorter bed rest durations (95% CI: 1.32–4.56)[16] . Extended immobilization impairs the lower limb muscle pump function, forcing venous return to rely predominantly on respiration and arterial pulsations—mechanisms insufficient to maintain normal blood flow velocity. Consequently, venous blood flow in the lower limbs decreases markedly, reaching 20%-30% of baseline levels[17] . This prolonged stasis significantly increases the likelihood of thrombus formation. Furthermore, in patients with lower limb burns, localized venous compression and inflammatory responses exacerbate DVT incidence, rising to 67.8% compared to 42.1% in non-lower limb burn cases[18] . This highlights that prolonged bed rest not only elevates DVT risk through hemodynamic disturbances but may also synergize with local burn-related factors to amplify thrombogenesis. Therefore, aggressive preventive measures, such as early rehabilitation exercises and mechanical prophylaxis, should be prioritized for immobilized burn patients, particularly those with lower limb injuries, to mitigate DVT risk.
Iatrogenic Risks of Central Venous Catheters (CVCs)
The use of central venous catheters (CVCs) significantly elevates the risk of deep vein thrombosis (DVT) in burn patients[19] . Studies reveal that CVC placement increases DVT risk by 2.15-fold (95% CI: 1.24–3.72), with femoral vein catheters associated with a notably higher DVT incidence (8.5%) compared to internal jugular vein catheters (0%)[20] . This disparity stems from anatomical and hemodynamic characteristics: femoral veins exhibit slower blood flow and are more prone to compression during limb movement, whereas the internal jugular vein benefits from faster flow rates and reduced external compression[21] .Pathologically, catheter insertion directly damages vascular endothelium, exposing collagen and triggering platelet aggregation, which initiates the coagulation cascade. Concurrently, localized inflammatory responses amplify prothrombotic mechanisms. Specifically, endothelial injury post-insertion activates platelets, prompting the release of procoagulant substances such as platelet factor 4 (PF4) and thromboxane A2 (TXA2). These mediators further activate clotting factors, fostering microthrombus formation. Additionally, inflammation induces endothelial cells to release cytokines like interleukin-1β (IL-1β) and C-reactive protein (CRP), which perpetuate coagulation activation, creating a vicious cycle that exacerbates thrombosis[22] .
Therefore, for burn patients requiring CVCs, prioritizing internal jugular vein access and strictly limiting catheter dwell time are critical to minimizing DVT risk. Concurrent monitoring of coagulation profiles (e.g., D-dimer levels) is essential for early detection and intervention in thrombotic events.
Prophylactic Efficacy and Clinical Optimization of Low-Molecular-Weight Heparin (LMWH)
This study confirms that low-molecular-weight heparin (LMWH) significantly reduces the incidence of deep vein thrombosis (DVT) in burn patients (prophylaxis group vs. non-prophylaxis group: 10.4% vs. 24.3%, P<0.01), with its protective effect further validated by multivariate analysis (OR=0.36, P=0.004)[23] . Mechanistically, LMWH exerts its antithrombotic effects through three pathways: Inhibition of coagulation factor Xa, blocking thrombin generation; Attenuation of systemic inflammatory responses, reducing the release of endothelial injury-associated mediators (e.g., TNF-α, IL-6); Stabilization of vascular endothelial function, inhibiting apoptosis and maintaining barrier integrity[24] . However, the LMWH prophylaxis coverage rate in this study was only 42.19%, indicating underutilization or suboptimal timing in clinical practice.
Based on the evidence, the following clinical optimization strategies are proposed:
Early intervention in high-risk populations: For patients with burns ≥30% TBSA, concurrent infections, or prolonged immobilization, initiate LMWH within 48 hours of admission (dose-adjusted by body weight and renal function).Dynamic monitoring and multidisciplinary collaboration: Combine D-dimer testing (threshold >1.5 μg/mL) with ultrasonographic screening, and coordinate individualized protocols among burn surgery, vascular surgery, and pharmacy teams.Balancing bleeding risks: For patients with renal insufficiency or bleeding tendencies, adopt reduced LMWH doses (e.g., enoxaparin 20-40 mg/day) and shorten treatment duration.
Future research should focus on defining the optimal LMWH dosing window, exploring its synergistic effects with mechanical prophylaxis, and validating safety in special populations (e.g., pediatric and elderly burn patients) through multicenter prospective studies.
Stratified Prevention Strategies
High-risk populations (burn size ≥30% TBSA, concurrent infection, or prolonged immobilization): Early initiation of low-molecular-weight heparin (LMWH) within 48 hours of admission, with dosage adjusted based on body weight and renal function[25] .Moderate-to-low-risk populations: Combined mechanical prophylaxis (e.g., intermittent pneumatic compression devices) and phased rehabilitation protocols (such as ankle pump exercises)[26] .
Optimized Value of Integrated Predictive Models
Single-factor models (e.g., burn size with AUC=0.72) exhibit limited predictive efficacy, whereas integrated models combining burn size, wound infection, prolonged immobilization, and CVC use demonstrate significantly improved accuracy (AUC=0.85, sensitivity 82.6%, specificity 80.3%). This model enables:Precise identification of high-risk patients: Enhanced anticoagulation therapy is required for those with a predicted probability ≥48%.Optimized resource allocation: Avoid overtreatment in low-risk populations.Dynamic monitoring: Early intervention is achievable through combined D-dimer testing (threshold >1.5 μg/mL) and weekly ultrasonographic screening.
Study Limitations and Future Directions
This single-center retrospective analysis has inherent limitations, including potential selection bias and the exclusion of laboratory parameters (e.g., D-dimer levels). Future research should prioritize multicenter prospective studies and explore the following:Machine learning models: Develop dynamic risk prediction systems by integrating clinical data, inflammatory biomarkers, and real-time activity monitoring.
Catheter site stratification: Clarify thrombotic risk differences between internal jugular and femoral vein catheterization.Multidisciplinary collaboration: Establish personalized anticoagulation protocols through coordinated efforts among burn surgery, vascular surgery, and pharmacy teams.
Conclusion
This study systematically delineates the multifactorial pathogenesis of deep vein thrombosis (DVT) in burn patients, emphasizing the synergistic interplay of extensive burns (≥30% TBSA), wound infection, prolonged immobilization, and central venous catheterization. The integration of these risk factors into a multidimensional predictive model (AUC=0.85) significantly enhances clinical risk stratification, enabling targeted interventions such as early low-molecular-weight heparin (LMWH) prophylaxis for high-risk populations (predicted probability ≥48%) and optimized resource allocation through dynamic monitoring (D-dimer >1.5 μg/mL and weekly ultrasonography). Despite limitations inherent to its single-center retrospective design, the findings underscore the necessity of adopting stratified prevention strategies and multidisciplinary collaboration to mitigate thrombotic complications. Future efforts should focus on validating these insights in multicenter prospective cohorts, refining machine learning-driven dynamic models, and establishing catheter site-specific protocols to advance precision medicine in burn care. Ultimately, bridging mechanistic insights with clinical implementation holds promise for reducing DVT-related morbidity and improving long-term outcomes in this vulnerable population.
Acknowledgements
Not Applicable.
Author contributions
WU Yindong: Study Conceptualization and Design: Proposed the research hypothesis, designed the retrospective cohort study framework, and established inclusion/exclusion criteria. Data Acquisition and Processing: Independently performed case screening, data extraction (via the Hospital Information System, HIS), database construction, and validated data integrity through rigorous cleaning procedures. Statistical Analysis: Conducted univariate analysis, multivariate logistic regression, and ROC curve analysis using SPSS 26.0 and R. Manuscript Drafting: Wrote the initial draft, including the Methods, Results, Discussion, and Conclusion sections, and created figures and tables (e.g., technical workflow diagrams, ROC curves). Model Development: Led the development of the multidimensional predictive model, authored validation code, and optimized sensitivity and specificity parameters.
HU Delin: Academic Supervision: Provided academic guidance on study design and reviewed the scientific validity of inclusion criteria and statistical methodologies. Manuscript Revision: Offered critical revisions to mechanistic interpretations, clinical implications, and limitations in the Discussion section. Resource Coordination: Facilitated access to clinical data and coordinated multi-departmental collaboration (e.g., Ultrasound Department, Laboratory Medicine). Quality Assurance: Oversaw ethical compliance and ensured the clinical applicability of conclusions.
Ethics approval and consent to participate
Not Applicable.
Funding information
Not Applicable.
Competing Interests
The authors declare that they have no existing or potential commercial or financial relationships that could create a conflict of interest at the time of conducting this study.
Data Availability
All data needed to evaluate the conclusions in the paper are present in the paper or the Supplementary Materials. Additional data related to this paper may be requested from the authors.
References
[1] Akl, E. A., Ramly, E. P., Kahale, L. A., Yosuico, V. E. D., Barba, M., Sperati, F.,et al. (2014). Anticoagulation for people with cancer and central venous catheters. The Cochrane Database of Systematic Reviews, 10, CD006468. https://doi.org/10.1002/14651858.CD006468.pub5
[2] Ball, R. L., Keyloun, J. W., Brummel-Ziedins, K., Orfeo, T., Palmieri, T. L., Johnson, L. S., et al. (2020). Burn-Induced Coagulopathies: A Comprehensive Review. Shock (Augusta, Ga.), 54(2), 154–167. https://doi.org/10.1097/SHK.0000000000001484
[3] Bordeanu-Diaconescu, E.-M., Grosu-Bularda, A., Frunza, A., Grama, S., Andrei, M.-C., Neagu, T. P., et al. (2024). Venous Thromboembolism in Burn Patients: A 5-Year Retrospective Study. Medicina (Kaunas, Lithuania), 60(2), 258. https://doi.org/10.3390/medicina60020258
[4] Chen, X., Wang, K., Li, D., Zhao, M., Huang, B., Su, W., et al. (2022). Genetic and immune crosstalk between severe burns and blunt trauma: A study of transcriptomic data. Frontiers in Genetics, 13, 1038222. https://doi.org/10.3389/fgene.2022.1038222
[5] CRISTAL Study Group, Sidhu, V. S., Kelly, T.-L., Pratt, N., Graves, S. E., Buchbinder, R., Adie, S., et al. (2022). Effect of Aspirin vs Enoxaparin on Symptomatic Venous Thromboembolism in Patients Undergoing Hip or Knee Arthroplasty: The CRISTAL Randomized Trial. JAMA, 328(8), 719–727. https://doi.org/10.1001/jama.2022.13416
[6] Department of Anesthesia and Intensive Care, Emergency Clinical Hospital of Bucharest, Romania, Tiglis, M., Peride, I., Department of Nephrology, ”Carol Davila” University of Medicine and Pharmacy Bucharest, Romania, Neagu, T. P., Department of Plastic Surgery and Reconstructive Microsurgery, ”Carol Davila” University of Medicine and Pharmacy Bucharest, Romania, Niculae, A., Department of Nephrology, ”Carol Davila” University of Medicine and Pharmacy Bucharest, Romania, Lascar, I., & Department of Plastic Surgery and Reconstructive Microsurgery, ”Carol Davila” University of Medicine and Pharmacy Bucharest, Romania. (2022). Venous thromboembolism in burn patients – low incidence or underdiagnosis? Romanian Journal of Medical Practice, 17(4), 164–167. https://doi.org/10.37897/RJMP.2022.4.5
[7] Ding, K., Yan, W., Zhang, Y., Li, J., Li, C., & Liang, C. (2024). The safety and efficacy of NOACs versus LMWH for thromboprophylaxis after THA or TKA: A systemic review and meta-analysis. Asian Journal of Surgery, 47(10), 4260–4270. https://doi.org/10.1016/j.asjsur.2024.02.113
[8] Eck, R. J., Bult, W., Wetterslev, J., Gans, R. O. B., Meijer, K., Keus, F., et al. (2019). Intermediate Dose Low-Molecular-Weight Heparin for Thrombosis Prophylaxis: Systematic Review with Meta-Analysis and Trial Sequential Analysis. Seminars in Thrombosis and Hemostasis, 45(8), 810–824. https://doi.org/10.1055/s-0039-1696965
[9] Ho, K. M., Bham, E., & Pavey, W. (2015). Incidence of Venous Thromboembolism and Benefits and Risks of Thromboprophylaxis After Cardiac Surgery: A Systematic Review and Meta-Analysis. Journal of the American Heart Association, 4(10), e002652. https://doi.org/10.1161/JAHA.115.002652
[10] Jackson, B. S., & Pretorius, E. (2019). Pathological Clotting and Deep Vein Thrombosis in Patients with HIV. Seminars in Thrombosis and Hemostasis, 45(2), 132–140. https://doi.org/10.1055/s-0038-1676374
[11] Jayarajah, U., Basnayake, O., & Ekanayake, G. (2020). Critical care management in burns: A review of current evidence and guidelines - Part 2. Sri Lanka Journal of Surgery, 38(3). https://doi.org/10.4038/sljs.v38i3.8788
[12] Kruger, P. C., Eikelboom, J. W., Douketis, J. D., & Hankey, G. J. (2019). Deep vein thrombosis: Update on diagnosis and management. The Medical Journal of Australia, 210(11), 516–524. https://doi.org/10.5694/mja2.50201
[13] Lee, Y., Jehangir, Q., Li, P., Gudimella, D., Mahale, P., Lin, C.-H., et al. (2022). Venous thromboembolism in COVID-19 patients and prediction model: A multicenter cohort study. BMC Infectious Diseases, 22(1), 462. https://doi.org/10.1186/s12879-022-07421-3
[14] Li, D., Yuan, M., & Yang, H. (2022). Blood Cell Parameters Combined with Inflammatory Markers in the Early Diagnosis of Pulmonary Embolism. Cellular and Molecular Biology (Noisy-Le-Grand, France), 68(5), 177–185. https://doi.org/10.14715/cmb/2022.68.5.24
[15] Lu, R. P., Lin, F.-C., Ortiz-Pujols, S. M., Adams, S. D., Whinna, H. C., Cairns, B. A., et al. (2013). Blood utilization in patients with burn injury and association with clinical outcomes (CME). Transfusion, 53(10), 2212–2221; quiz 2211. https://doi.org/10.1111/trf.12057
[16] Pannucci, C. J., Osborne, N. H., & Wahl, W. L. (2011). Venous thromboembolism in thermally injured patients: Analysis of the National Burn Repository. Journal of Burn Care & Research: Official Publication of the American Burn Association, 32(1), 6–12. https://doi.org/10.1097/BCR.0b013e318204b2ff
[17] Peng, G., Wang, Q., Sun, H., Gan, L., Lu, H., Deng, Z., et al. (2021). Development and prospective validation of a novel risk score for predicting the risk of lower extremity deep vein thrombosis among multiple trauma patients. Thrombosis Research, 201, 116–122. https://doi.org/10.1016/j.thromres.2021.02.020
[18] Qian, X., Ge, Y., & Luo, J. (2024). Risk factors for deep vein thrombosis after traumatic lower extremity fracture: A systematic review and meta-analysis. Medicine, 103(23), e38439. https://doi.org/10.1097/MD.0000000000038439
[19] Roldan, C. J., & Paniagua, L. (2015). Central Venous Catheter Intravascular Malpositioning: Causes, Prevention, Diagnosis, and Correction. The Western Journal of Emergency Medicine, 16(5), 658–664. https://doi.org/10.5811/westjem.2015.7.26248
[20] Stanton, E. W., Manasyan, A., Thompson, C. M., Patel, G. P., Lacey, A. M., Travis, T. E., et al. (2024). Venous Thromboembolism Incidence, Risk Factors, and Prophylaxis in Burn Patients: A National Trauma Database Study. Journal of Burn Care & Research: Official Publication of the American Burn Association, irae171. https://doi.org/10.1093/jbcr/irae171
[21] Teja, B., Bosch, N. A., Diep, C., Pereira, T. V., Mauricio, P., Sklar, M. C., et al. (2024). Complication Rates of Central Venous Catheters: A Systematic Review and Meta-Analysis. JAMA Internal Medicine, 184(5), 474–482. https://doi.org/10.1001/jamainternmed.2023.8232
[22] Toker, S., Hak, D. J., & Morgan, S. J. (2011). Deep vein thrombosis prophylaxis in trauma patients. Thrombosis, 2011, 505373. https://doi.org/10.1155/2011/505373
[23] Tullie, S., Nicholson, T., Bishop, J. R. B., McGee, K. C., Asiri, A., Sullivan, J., et al. (2024). Severe thermal and major traumatic injury results in elevated plasma concentrations of total heme that are associated with poor clinical outcomes and systemic immune suppression. Frontiers in Immunology, 15, 1416820. https://doi.org/10.3389/fimmu.2024.1416820
[24] van Minnen, O., van den Bergh, W. M., Droogh, J. M., Koehorst, L., Lagrand, W. K., Raasveld, S. J., et al. (2022). Incidence and risk factors of deep vein thrombosis after extracorporeal life support. Artificial Organs, 46(9), 1893–1900. https://doi.org/10.1111/aor.14271
[25] Wei, Q., Wei, Z.-Q., Jing, C.-Q., Li, Y.-X., Zhou, D.-B., Lin, M.-B., et al. (2023). Incidence, prevention, risk factors, and prediction of venous thromboembolism in Chinese patients after colorectal cancer surgery: A prospective, multicenter cohort study. International Journal of Surgery (London, England), 109(10), 3003–3012. https://doi.org/10.1097/JS9.0000000000000553
[26] Yang, X., Li, H., Yu, L., Yu, T., Li, F., Yu, Z., et al. (2021). [Analysis of Influencing Factors for Postoperative Venous Thromboembolism of Thymic Malignancies]. Zhongguo Fei Ai Za Zhi = Chinese Journal of Lung Cancer, 24(7), 497–502. https://doi.org/10.3779/j.issn.1009-3419.2021.101.22
 Figures
Figures References
References Peer
Peer Information
InformationFigure1. ROC Curve. The integrative model synthesizing burn surface area, infectious wound status, prolonged immobilization period, and central venous catheter utilization exhibited significantly enhanced predictive accuracy relative to univariate analytical approaches.

[1] Akl, E. A., Ramly, E. P., Kahale, L. A., Yosuico, V. E. D., Barba, M., Sperati, F.,et al. (2014). Anticoagulation for people with cancer and central venous catheters. The Cochrane Database of Systematic Reviews, 10, CD006468. https://doi.org/10.1002/14651858.CD006468.pub5
[2] Ball, R. L., Keyloun, J. W., Brummel-Ziedins, K., Orfeo, T., Palmieri, T. L., Johnson, L. S., et al. (2020). Burn-Induced Coagulopathies: A Comprehensive Review. Shock (Augusta, Ga.), 54(2), 154–167. https://doi.org/10.1097/SHK.0000000000001484
[3] Bordeanu-Diaconescu, E.-M., Grosu-Bularda, A., Frunza, A., Grama, S., Andrei, M.-C., Neagu, T. P., et al. (2024). Venous Thromboembolism in Burn Patients: A 5-Year Retrospective Study. Medicina (Kaunas, Lithuania), 60(2), 258. https://doi.org/10.3390/medicina60020258
[4] Chen, X., Wang, K., Li, D., Zhao, M., Huang, B., Su, W., et al. (2022). Genetic and immune crosstalk between severe burns and blunt trauma: A study of transcriptomic data. Frontiers in Genetics, 13, 1038222. https://doi.org/10.3389/fgene.2022.1038222
[5] CRISTAL Study Group, Sidhu, V. S., Kelly, T.-L., Pratt, N., Graves, S. E., Buchbinder, R., Adie, S., et al. (2022). Effect of Aspirin vs Enoxaparin on Symptomatic Venous Thromboembolism in Patients Undergoing Hip or Knee Arthroplasty: The CRISTAL Randomized Trial. JAMA, 328(8), 719–727. https://doi.org/10.1001/jama.2022.13416
[6] Department of Anesthesia and Intensive Care, Emergency Clinical Hospital of Bucharest, Romania, Tiglis, M., Peride, I., Department of Nephrology, ”Carol Davila” University of Medicine and Pharmacy Bucharest, Romania, Neagu, T. P., Department of Plastic Surgery and Reconstructive Microsurgery, ”Carol Davila” University of Medicine and Pharmacy Bucharest, Romania, Niculae, A., Department of Nephrology, ”Carol Davila” University of Medicine and Pharmacy Bucharest, Romania, Lascar, I., & Department of Plastic Surgery and Reconstructive Microsurgery, ”Carol Davila” University of Medicine and Pharmacy Bucharest, Romania. (2022). Venous thromboembolism in burn patients – low incidence or underdiagnosis? Romanian Journal of Medical Practice, 17(4), 164–167. https://doi.org/10.37897/RJMP.2022.4.5
[7] Ding, K., Yan, W., Zhang, Y., Li, J., Li, C., & Liang, C. (2024). The safety and efficacy of NOACs versus LMWH for thromboprophylaxis after THA or TKA: A systemic review and meta-analysis. Asian Journal of Surgery, 47(10), 4260–4270. https://doi.org/10.1016/j.asjsur.2024.02.113
[8] Eck, R. J., Bult, W., Wetterslev, J., Gans, R. O. B., Meijer, K., Keus, F., et al. (2019). Intermediate Dose Low-Molecular-Weight Heparin for Thrombosis Prophylaxis: Systematic Review with Meta-Analysis and Trial Sequential Analysis. Seminars in Thrombosis and Hemostasis, 45(8), 810–824. https://doi.org/10.1055/s-0039-1696965
[9] Ho, K. M., Bham, E., & Pavey, W. (2015). Incidence of Venous Thromboembolism and Benefits and Risks of Thromboprophylaxis After Cardiac Surgery: A Systematic Review and Meta-Analysis. Journal of the American Heart Association, 4(10), e002652. https://doi.org/10.1161/JAHA.115.002652
[10] Jackson, B. S., & Pretorius, E. (2019). Pathological Clotting and Deep Vein Thrombosis in Patients with HIV. Seminars in Thrombosis and Hemostasis, 45(2), 132–140. https://doi.org/10.1055/s-0038-1676374
[11] Jayarajah, U., Basnayake, O., & Ekanayake, G. (2020). Critical care management in burns: A review of current evidence and guidelines - Part 2. Sri Lanka Journal of Surgery, 38(3). https://doi.org/10.4038/sljs.v38i3.8788
[12] Kruger, P. C., Eikelboom, J. W., Douketis, J. D., & Hankey, G. J. (2019). Deep vein thrombosis: Update on diagnosis and management. The Medical Journal of Australia, 210(11), 516–524. https://doi.org/10.5694/mja2.50201
[13] Lee, Y., Jehangir, Q., Li, P., Gudimella, D., Mahale, P., Lin, C.-H., et al. (2022). Venous thromboembolism in COVID-19 patients and prediction model: A multicenter cohort study. BMC Infectious Diseases, 22(1), 462. https://doi.org/10.1186/s12879-022-07421-3
[14] Li, D., Yuan, M., & Yang, H. (2022). Blood Cell Parameters Combined with Inflammatory Markers in the Early Diagnosis of Pulmonary Embolism. Cellular and Molecular Biology (Noisy-Le-Grand, France), 68(5), 177–185. https://doi.org/10.14715/cmb/2022.68.5.24
[15] Lu, R. P., Lin, F.-C., Ortiz-Pujols, S. M., Adams, S. D., Whinna, H. C., Cairns, B. A., et al. (2013). Blood utilization in patients with burn injury and association with clinical outcomes (CME). Transfusion, 53(10), 2212–2221; quiz 2211. https://doi.org/10.1111/trf.12057
[16] Pannucci, C. J., Osborne, N. H., & Wahl, W. L. (2011). Venous thromboembolism in thermally injured patients: Analysis of the National Burn Repository. Journal of Burn Care & Research: Official Publication of the American Burn Association, 32(1), 6–12. https://doi.org/10.1097/BCR.0b013e318204b2ff
[17] Peng, G., Wang, Q., Sun, H., Gan, L., Lu, H., Deng, Z., et al. (2021). Development and prospective validation of a novel risk score for predicting the risk of lower extremity deep vein thrombosis among multiple trauma patients. Thrombosis Research, 201, 116–122. https://doi.org/10.1016/j.thromres.2021.02.020
[18] Qian, X., Ge, Y., & Luo, J. (2024). Risk factors for deep vein thrombosis after traumatic lower extremity fracture: A systematic review and meta-analysis. Medicine, 103(23), e38439. https://doi.org/10.1097/MD.0000000000038439
[19] Roldan, C. J., & Paniagua, L. (2015). Central Venous Catheter Intravascular Malpositioning: Causes, Prevention, Diagnosis, and Correction. The Western Journal of Emergency Medicine, 16(5), 658–664. https://doi.org/10.5811/westjem.2015.7.26248
[20] Stanton, E. W., Manasyan, A., Thompson, C. M., Patel, G. P., Lacey, A. M., Travis, T. E., et al. (2024). Venous Thromboembolism Incidence, Risk Factors, and Prophylaxis in Burn Patients: A National Trauma Database Study. Journal of Burn Care & Research: Official Publication of the American Burn Association, irae171. https://doi.org/10.1093/jbcr/irae171
[21] Teja, B., Bosch, N. A., Diep, C., Pereira, T. V., Mauricio, P., Sklar, M. C., et al. (2024). Complication Rates of Central Venous Catheters: A Systematic Review and Meta-Analysis. JAMA Internal Medicine, 184(5), 474–482. https://doi.org/10.1001/jamainternmed.2023.8232
[22] Toker, S., Hak, D. J., & Morgan, S. J. (2011). Deep vein thrombosis prophylaxis in trauma patients. Thrombosis, 2011, 505373. https://doi.org/10.1155/2011/505373
[23] Tullie, S., Nicholson, T., Bishop, J. R. B., McGee, K. C., Asiri, A., Sullivan, J., et al. (2024). Severe thermal and major traumatic injury results in elevated plasma concentrations of total heme that are associated with poor clinical outcomes and systemic immune suppression. Frontiers in Immunology, 15, 1416820. https://doi.org/10.3389/fimmu.2024.1416820
[24] van Minnen, O., van den Bergh, W. M., Droogh, J. M., Koehorst, L., Lagrand, W. K., Raasveld, S. J., et al. (2022). Incidence and risk factors of deep vein thrombosis after extracorporeal life support. Artificial Organs, 46(9), 1893–1900. https://doi.org/10.1111/aor.14271
[25] Wei, Q., Wei, Z.-Q., Jing, C.-Q., Li, Y.-X., Zhou, D.-B., Lin, M.-B., et al. (2023). Incidence, prevention, risk factors, and prediction of venous thromboembolism in Chinese patients after colorectal cancer surgery: A prospective, multicenter cohort study. International Journal of Surgery (London, England), 109(10), 3003–3012. https://doi.org/10.1097/JS9.0000000000000553
[26] Yang, X., Li, H., Yu, L., Yu, T., Li, F., Yu, Z., et al. (2021). [Analysis of Influencing Factors for Postoperative Venous Thromboembolism of Thymic Malignancies]. Zhongguo Fei Ai Za Zhi = Chinese Journal of Lung Cancer, 24(7), 497–502. https://doi.org/10.3779/j.issn.1009-3419.2021.101.22
Peer-review Terminology
Identity transparency: Single anonymized
Reviewer interacts with: Editor
Review information published:
Review reports
Reviewer identities if reviewer opts in
Author/reviewer communication
Details
© 2025 The Author(s). Cell Conflux published by Life Conflux Press Limited on behalf of Conflux Science.
This is an open access article under the terms of the Creative Commons Attribution License, which permits use, distribution and reproduction in any medium, provided the original work is properly cited.
Publication History
Received 2025-03-15
Accepted 2025-04-30
Published 2025-05-14


